FINDING SOLUTIONS
Suppliers are taking on the challenges of blister packaging with innovations in machinery and materials.
By Bobby Douglas
For the convenience and ease of use blister packaging provides consumers, the creation of such packaging isn’t as simple. From developing appropriate machinery to sourcing the right materials, blister packaging brings a variety of challenges that suppliers are constantly trying to mitigate.
As an example, Pharmaworks has developed blister machinery that can handle 100% recyclable mono-materials, marking a significant improvement when it comes to the sustainability of these operations.
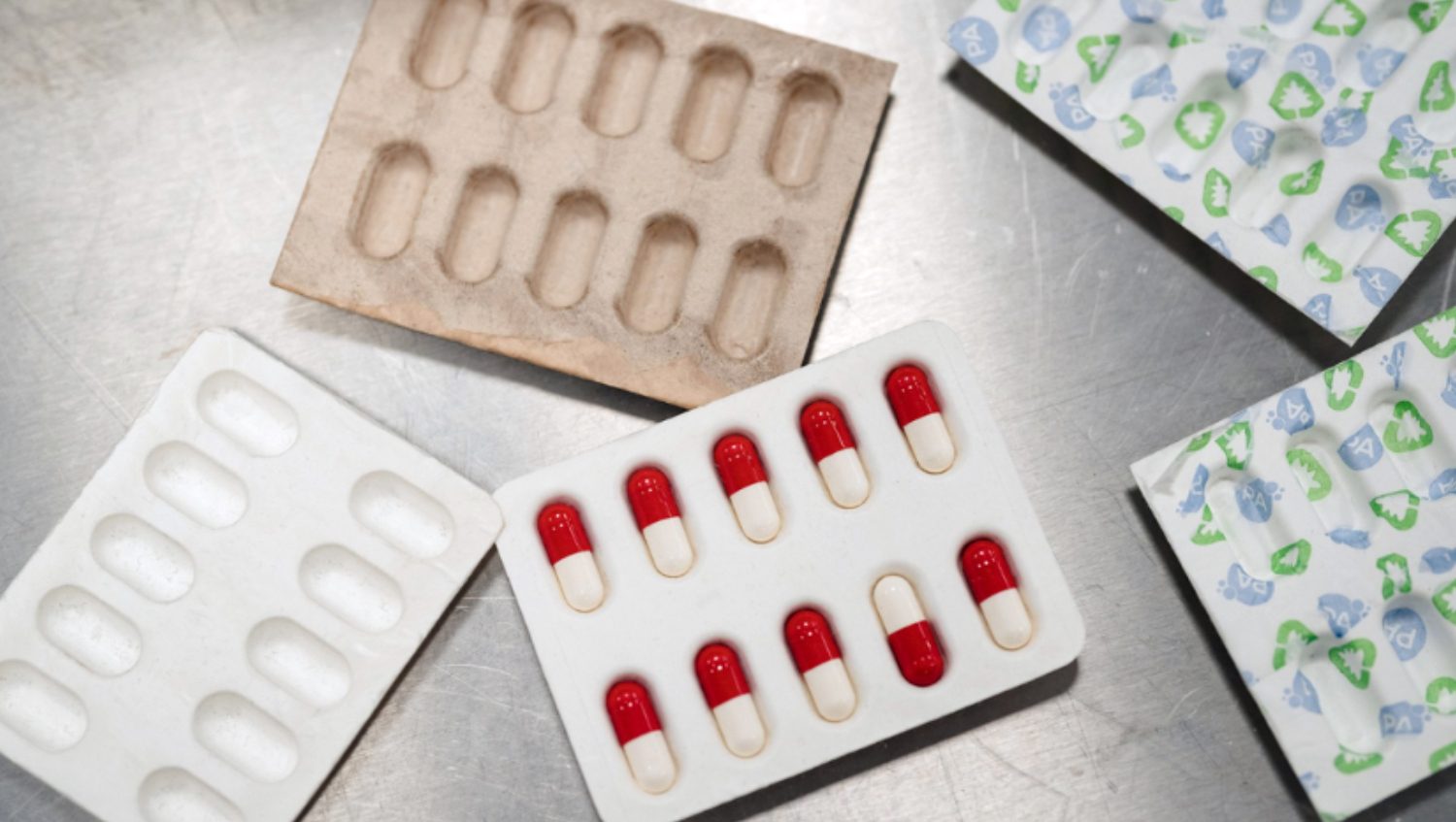
Additionally, PulPac, PA Consulting and Optima collaborated to develop bespoke machinery that can create blister packaging from Dry Molded Fiber. The collaboration allows this practice to operate at a large scale while also contributing to a circular economy.
Finally, Aptar CSP Technologies announced that its N-Sorb nitrosamine mitigation solution was accepted into the U.S. Food & Drug Administration’s Emerging Technology Program, which promotes the adoption of innovative approaches to pharmaceutical product design and manufacturing.
Learn more about these innovations below.
ADVERTISEMENT
Pharmaworks Develops Blister Machine for 100% Recyclable Mono-Materials
Pharmaworks, a ProMach product brand, recently debuted its new capability to handle recyclable, mono-material packaging films on its blister machines at Interphex. In addition to offering their entire fleet of available blister packaging systems ready to run these challenging films, the company is offering upgrades for installed Pharmaworks blister machines in the field and can rebuild blister machines from other suppliers to handle these new sustainable films.
READ MORE
“As the leading blister machine manufacturer in North America, we’re helping improve sustainability by ensuring our equipment is compatible with the new mono-base and mono-lid materials. Our readiness will enable domestic customers to quickly adopt these new films as they become available and will satisfy the immediate needs of customers elsewhere in the world, including Europe, where sustainability initiatives have already ushered in these mono-materials,” said Ben Brower, co-founder and current Director of Applications at Pharmaworks. “Our current blister packaging technology is so advanced, we’re able to handle the machinability challenges that these new films present without redesigning the equipment.”
Compared to traditional PVC, PVDC and multi-structure films, which are essentially non-recyclable, the new mono-structure films such as PP from Etimex and Liveo, PE from Amcor and PET from Liveo and Klockner Pentaplast are 100 percent recyclable. As mono-material suppliers work with their customers to deliver the barrier properties, shelf life, child-resistant/senior-friendly opening features and printability needed, equipment manufacturers are partnering with the material suppliers to ensure machinability.
After extensive trials with a variety of mono-structure films on the world market, Pharmaworks has confirmed their sophisticated blister machines are already equipped to address the challenges associated with difficult-to-handle mono-structure films. Compared to traditional blister materials, mono-structure films have a tighter heat tolerance and they tend to stretch after thermoforming. Also, sealing is harder because, without introducing an adhesive laminate, the pressure applied becomes more critical.
Each of Pharmaworks’ five blister machine models currently available features high-performance lasers, servo motors, precision tooling and software algorithms that allow the equipment to easily produce incredible results with mono-structure materials.
Pharmaworks can also upgrade existing blister machines to make them compatible with mono-materials.
Courtesy of Pharmaworks

COLLAPSE ARTICLE ABOVE
PulPac, PA Consulting and Optima Collaborate on Machinery for Dry Molded Fiber
PulPac, PA Consulting, and Optima have partnered to develop bespoke machinery for advanced Dry Molded Fiber applications.
The partnership aims to facilitate the industrialization of complex products such as coffee capsules and blister packaging. By integrating PulPac’s pioneering Dry Molded Fiber technology with PA Consulting’s product development expertise and Optima’s renowned bespoke machine building capabilities, the collaboration will drive the scalability and high-throughput production of commercially viable, advanced Dry Molded Fiber applications, supporting PulPac’s and PA Consulting’s Blister Pack Collective and other initiatives for advanced Dry Molded Fiber packaging.
READ MORE
There is a substantial market demand for eco-friendly alternatives in categories like coffee capsules and blister packaging. The new bespoke machinery will offer significant advantages for brands within those segments, providing them with the ability to integrate cost-efficient sustainable packaging solutions at scale. By expanding the range of what is possible with Dry Molded Fiber on an industrial scale, the collaboration will help brands enhance their sustainability efforts and meet evolving consumer expectations as well as legislative demands.
"To make progress in developing products like coffee capsules and blister packs, they must be manufacturable at scale," said Keith Thornley, Commercial Lead for Dry Molded Fiber Collectives at PA Consulting. "We're excited to work with PulPac and Optima to develop specialized machinery that will enable the high-volume production of complex Dry Molded Fiber products, driving significant advancements in the industry.”
Optima’s Director of Fiber Solutions, Dominik Bröllochs, added, “As a one-stop-shop partner for fiber-based packaging, we not only bring our expertise in customized machines to this collaboration, but also our curiosity to push the boundaries of what is possible in the field of sustainable packaging. By integrating optimized solutions for specific Dry Molded Fiber applications, such as the right barrier, we are setting new standards for sustainable products, meeting the demand of brand owners and converters.”
PulPac's Chief Operations Officer, Viktor Börjesson, concluded, "We have proven that Dry Molded Fiber can be used for making advanced products, but turning that potential into real-world impact requires scaling production to an industrial level. Through this collaboration, we enable brands to adopt more sustainable practices at scale — making a tangible difference in reducing reliance on non-renewable materials and promoting a circular economy.”
The collaboration allows for the use of sustainable materials while maintaining high-level production.
Courtesy of PulPac
COLLAPSE ARTICLE ABOVE
Aptar Earns Acceptance to FDA's Emerging Technology Program for N-Sorb Solution
Aptar CSP Technologies, part of AptarGroup, Inc. and a leader in active material science, announced that its N-Sorb nitrosamine mitigation solution has been accepted into the U.S. Food & Drug Administration’s (FDA) Emerging Technology Program (ETP), which helps promote the adoption of innovative approaches to pharmaceutical product design and manufacturing.
N-Sorb leverages Aptar CSP Technologies’ proven 3-Phase Activ-Polymer™ platform technology to address the pressing issue of N-nitrosamine impurities in pharmaceuticals. These impurities, classified as probable human carcinogens, have raised significant regulatory concerns and prompted numerous drug product recalls. Nitrosamines can form during drug product storage or transport, posing risks to patient health.
READ MORE
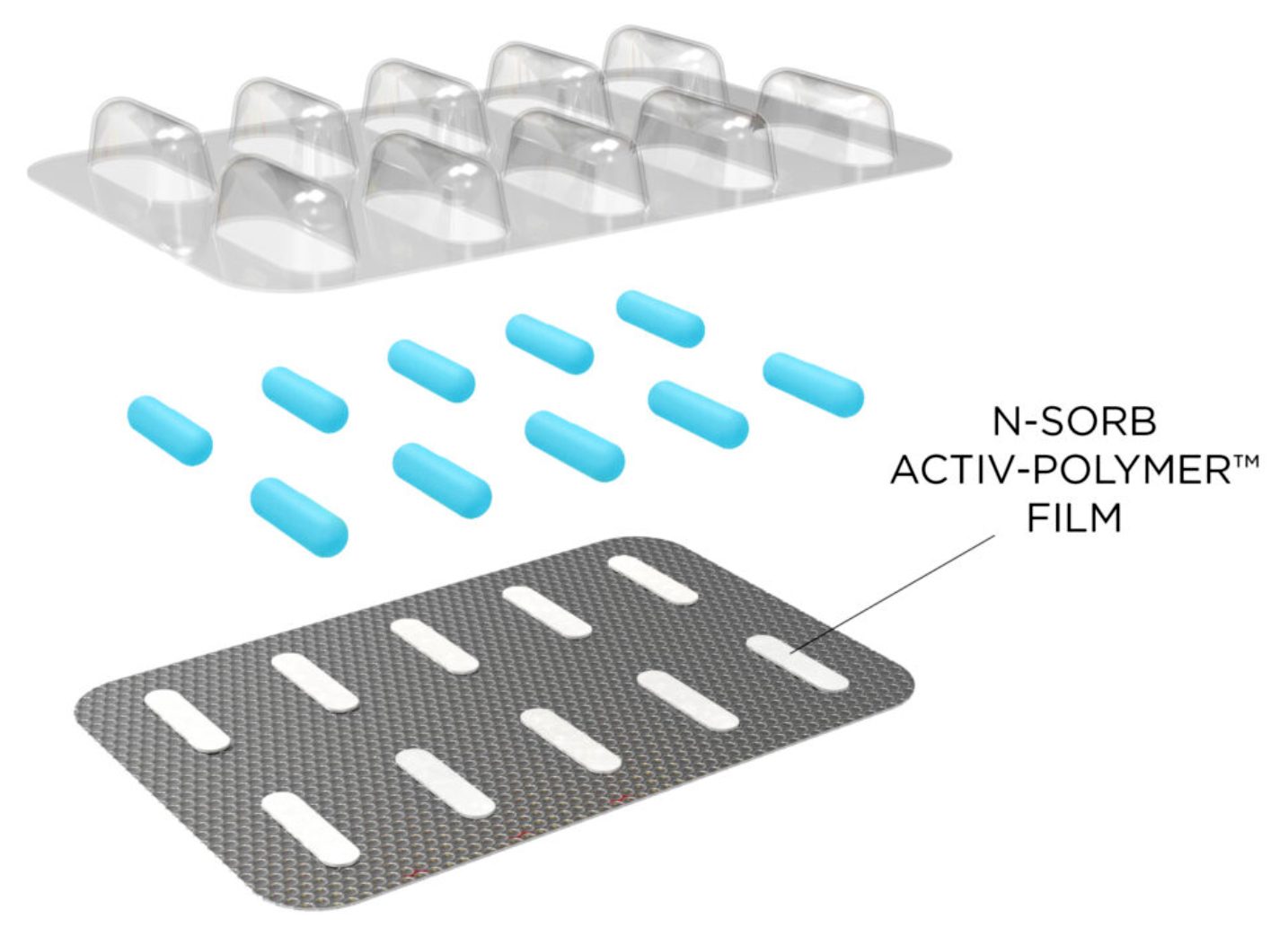
N-Sorb technology can be deployed in multiple formats by integrating active material science into polymers. For example, the technology can be incorporated into a blister format that integrates a piece of N-Sorb Activ-Film™ material into each individual Activ-Blister™ cavity. This same platform is currently trusted by global brands to protect sensitive Active Pharmaceutical Ingredients (APIs) from degradation due to moisture and oxygen exposure. Alternatively, the technology can be seamlessly integrated into a container closure system. N-Sorb’s intelligent design allows it to react with nitrosamine precursors in the packaging headspace to inhibit nitrosamine formation and scavenge nitrosamine impurities post-formation.
By delivering this Generally Recognized as Safe (GRAS) material directly within the packaging, N-Sorb can eliminate the need for pharmaceutical developers to reformulate their drug products, which could support compliance with US FDA and EU EMA regulations regarding safe nitrosamine levels. The active packaging intervention represents a paradigm shift in managing impurities and degradation, which could significantly enhance overall mitigation strategies and aligns with the latest FDA guidance (updated Sept. 4, 2024) that recognizes packaging changes as a potential mitigation strategy. By addressing nitrosamine concerns with active packaging, N-Sorb technology can help accelerate drug product development and help alleviate the burden of drug shortages due to recalls.
“The FDA’s Emerging Technology Program is highly selective, reserved for the most promising pharma and healthcare sector solutions,” said Badre Hammond, VP Global Commercial Operations and GM for Aptar CSP Technologies. “Our ability to mitigate nitrosamine formation with active material science introduces a critical quality control element designed to ensure patient safety. We are eager to collaborate with the FDA’s Emerging Technology team to empower pharma brands with this innovative offering.”
As part of the program, industry representatives meet with the FDA’s Emerging Technology Team members to discuss, identify and resolve potential technical and regulatory issues related to the development and implementation of novel technologies prior to regulatory submission. This multi-stakeholder effort aligns with the FDA’s mission to facilitate modernization in the pharmaceutical industry, reducing time and cost requirements for introducing novel solutions.